Despite the harmonized and centralized process for drug registration in six countries, the requirements for approval of drug products in major countries like Saudi Arabia and UAE are independent. These countries have their own regulatory systems and their prosecution. Different regulations apply in each GCC country.
Check the table below for KSA registration details.
Check the table below for KSA registration details.

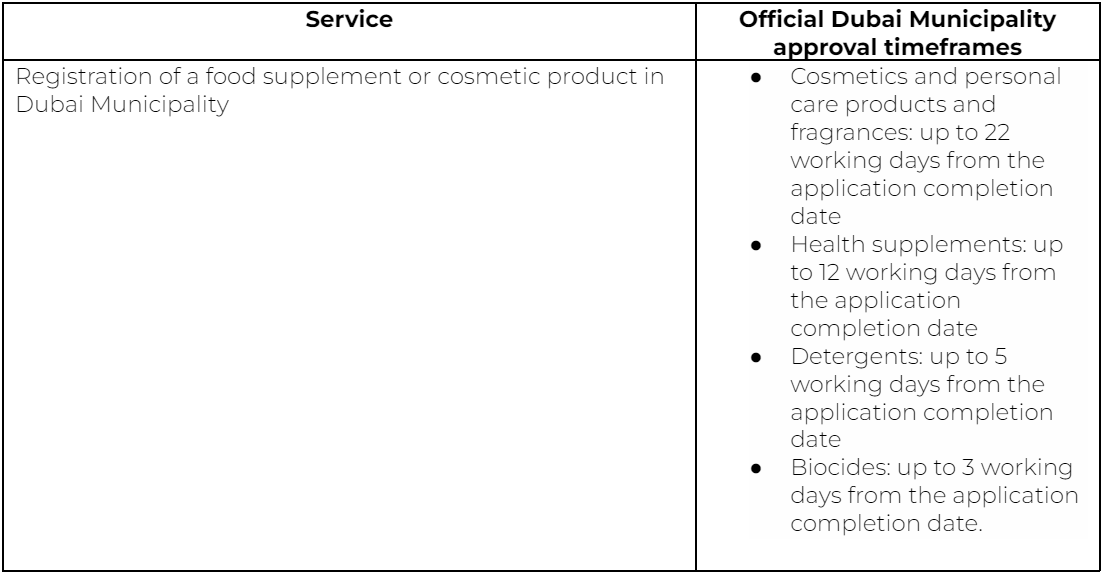
Check the table below for UAE registration details.
Marketing authorization validity period
The initial MA for consumer healthcare products is issued for 5 years.
Registration is done by a local legal entity which has acquired the necessary licenses. If you choose to register your products with us, we will act as the registration agent and hold the registration certificate (marketing authorization). If necessary and when possible, the MA can be transferred to another legal entity that owns the appropriate licenses. Fee of the marketing authorization holder to be discussed depending on the case.
The initial MA for consumer healthcare products is issued for 5 years.
Registration is done by a local legal entity which has acquired the necessary licenses. If you choose to register your products with us, we will act as the registration agent and hold the registration certificate (marketing authorization). If necessary and when possible, the MA can be transferred to another legal entity that owns the appropriate licenses. Fee of the marketing authorization holder to be discussed depending on the case.